NOTE: This features are only available with the Software Version 2.0.0.0 or higher.
From the Software Version 2.7.0.0 the HygroLab is FDA 21 CFR Part 11 and EU Annex 11 compliant. To be fully FDA 21 CFR Part 11 and EU Annex 11 compliant the user must carry out a validation, which can be done with the help of the IQ/OQ template (included in order code HYGROLAB-VAL-DOC), which can be purchased separately from Rotronic.
Warning: Once the HygroLab has been validated, the device cannot be updated. Otherwise, the entire device must be revalidated with the new version and with a new validation document (IQ/OQ template) suitable to the new HygroLab Software version.
Activating FDA Mode
Step 1 |
To get to the FDA mode or the FDA settings go to "Settings" > "Device settings" > "Local settings"

|
Step 2 |
Then click on the top the register which is labeled as "FDA", which is on the right side of the "Local settings".
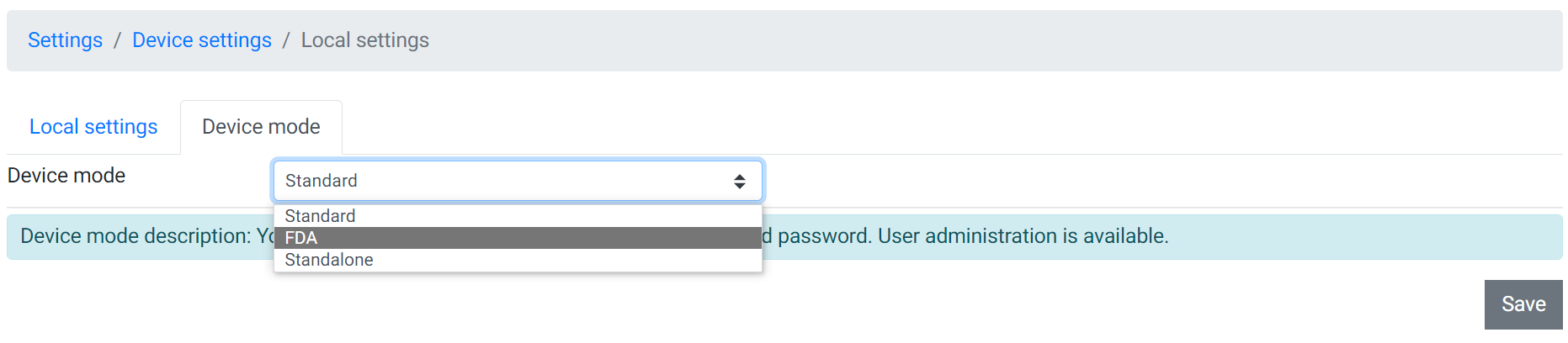 See more about the different HygroLab Modes here: General Settings
|
Step 3 |
The FDA settings can be only changed when the HygroLab is in the FDA mode. For that "Use FDA mode" needs to be enabled.After that the user can put (only) numbers into the input window. The X is a placeholder for a number.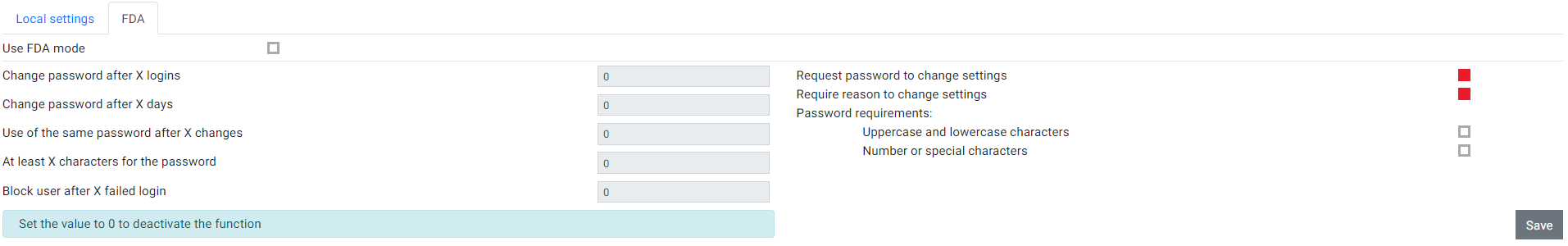 NOTE: User with "Manage user accounts" rights are not affected by the FDA Mode settings and when using LDAP function (the boxes are gray and can't be configured). |
Step 4 |
When everything is adjusted the way it should be click on the right side below the "Save" button to adopt the changes and to activate the FDA mode.The HygroLab shows on the top the FDA logo, when the device is in the FDA mode.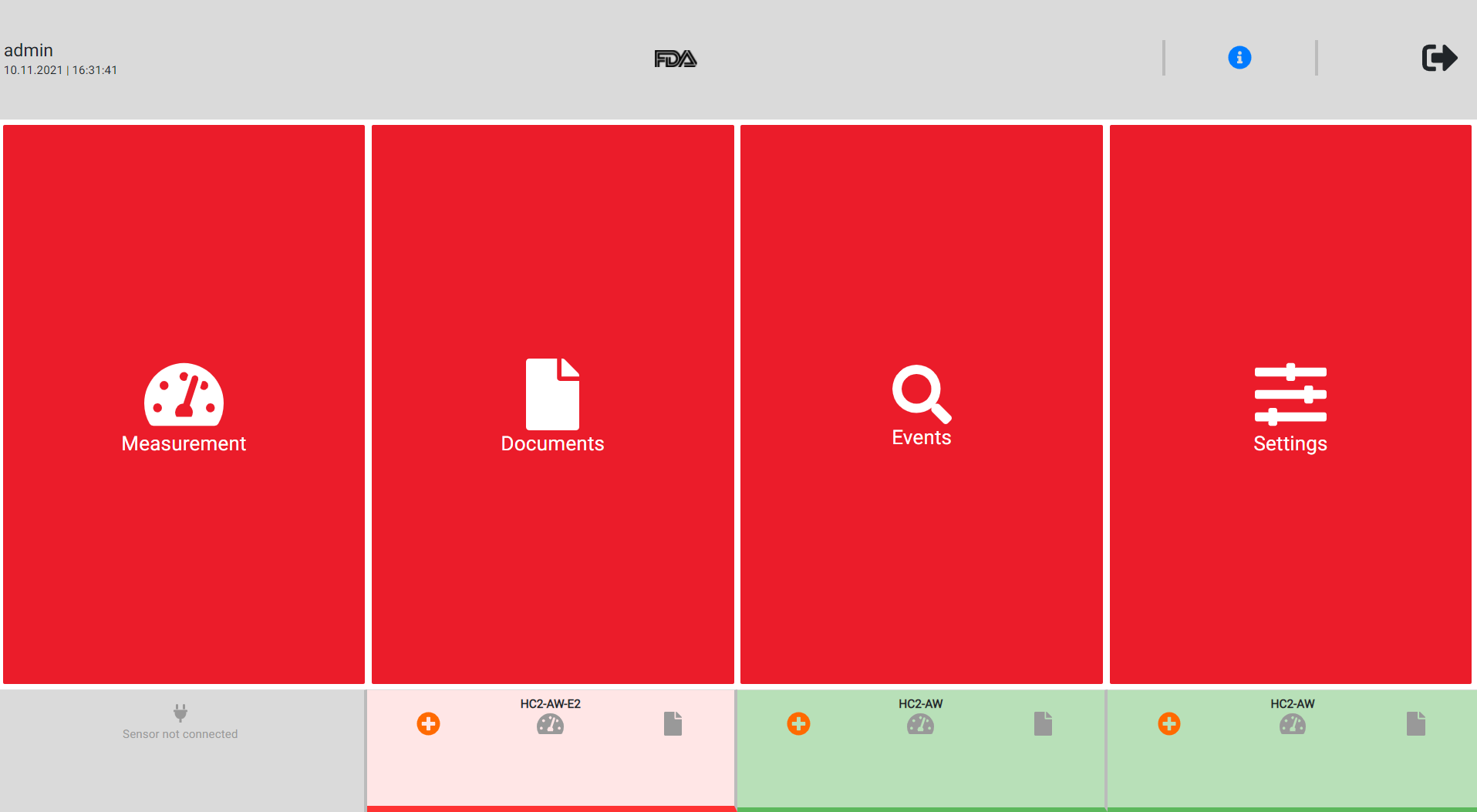 |