The mean kinetic temperature is the total influence of temperature on an object or product over a certain period of time.
Extract from the ICH Topic Q 1 A (R2): Stabilty Testing of new Drug Substances and Products:
Mean kinetic temperature: a single derived temperature that, if maintained over a defined period of time, affords the same thermal challenge to a drug substance or drug product as would be experienced over a range of both higher and lower temperatures for an equivalent defined period. The mean kinetic temperature is higher than the arithmetic mean temperature and takes into account the Arrhenius equation.
When establishing the mean kinetic temperature for a defined period, the formula of J. D. Haynes (J. Pharm. Sci., 60:927-929, 1971) can be used.
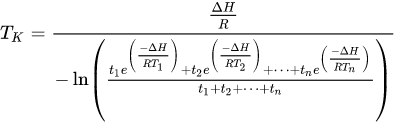
TK is the mean kinetic temperature in kelvin.
ΔH is the activation energy (in kJ mol-1).
R is the gas constant (in J mol-1 K-1).
T1 to TN are the temperature at each of the sample points in kelvin.
t1 to tn are the time intervals at each of the sample points.
The calculation in RMS is done with 32 bit precision, which is about 6 decimal places (including before the decimal point).
Update: 01.09.2023.